Ongoing Research Projects in the Schroeder Laboratory
Mouse neural-glial metabolic modeling for exploring the intersection of sleep and metabolism in neurological health
According to the astrocyte-neuron lactate shuttle hypothesis (ANLSH), strong metabolic coupling occurs between astrocytes and neurons, with the astrocyte serving “protective” and “supportive” roles for the neurons. Increased neural activity during “wake” periods, associated with glutamine “wake” signaling, leads to increased accumulation of fatty acids leading to increased production of reactive oxygen species (ROS) which induces lipid peroxidation, leading to disruption in neuronal membrane integrity. Astrocytes protect neurons from fatty acid- and excito- toxicity by the uptake of fatty acids and glutamate (produced during “wake” periods), particularly at the tripartite synapse, and shuttle lactate to neurons supporting wake-associated increased neural activity. It is hypothesized that sleep pressure builds with increased energy generation in neuronal mitochondria, in part through the export of ATP from astrocytes and somnogenic effect of adenosine, and the role of sleep is to synthesize lipids, repair and maintain cell membranes, and scavenge ROS. High lipid accumulation in astrocytes and dysregulation or disruption of lipid metabolism is associated with seizures, excitotoxicity, and epilepsy disease progression. This makes metabolic modeling an excellent tool for exploring the links between metabolism (primary and secondary), circadian rhythms, and neurological health. Particular diseases studied include epilepsy and traumatic brain injury (TBI).
Experimental Collaborator:Jason Gerstner
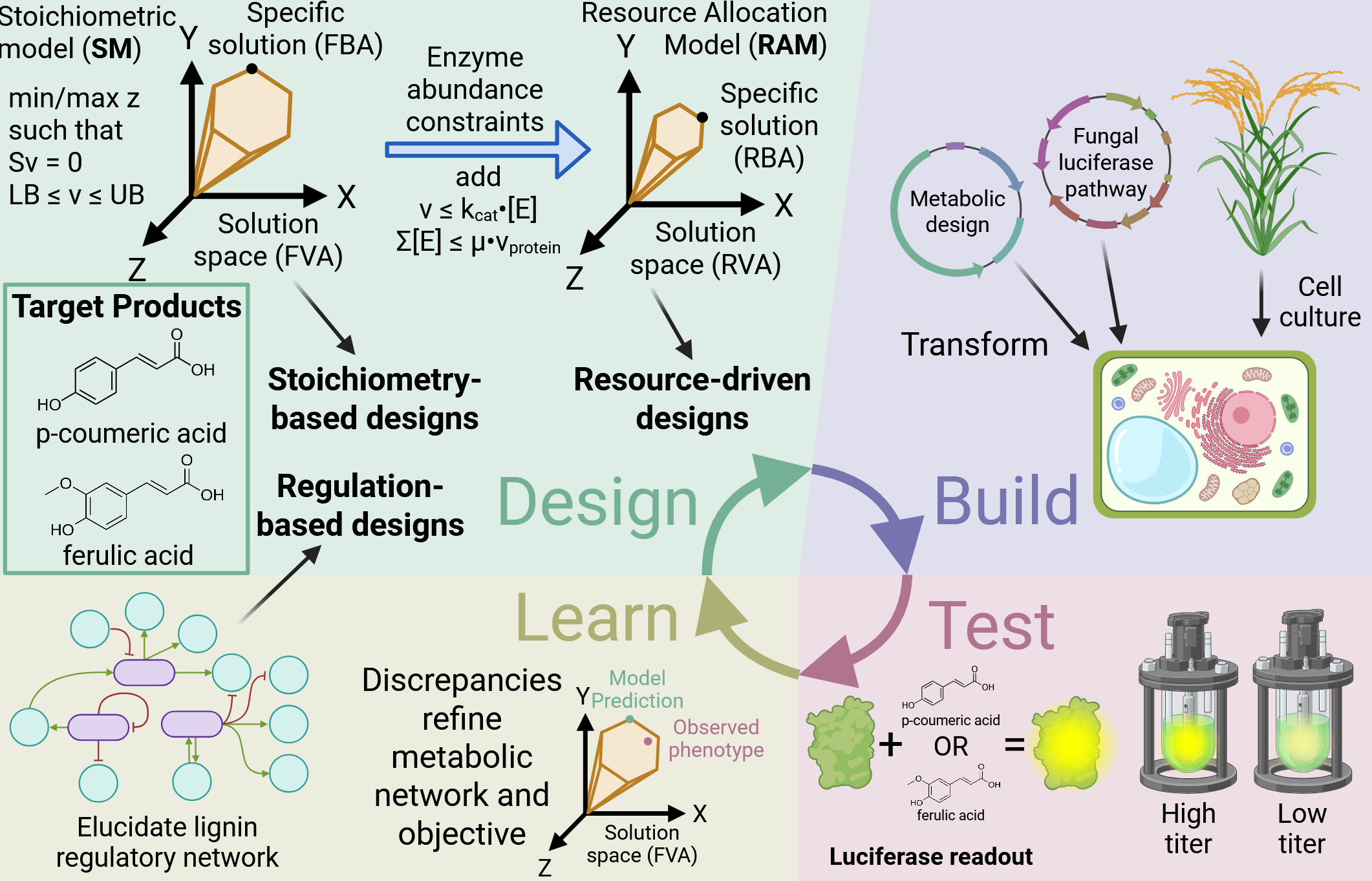
Rapid DBLT cycling for metabolic funneling of plant cells to produce aromatics
Lignin is heterogeneous in terms of bonds and cross linking, as well as monomers composition. These factors make deconstruction and subsequent valorization difficult, respectively, due to the need for a diverse array of chemical rearrangements to arrive at a desired product. A potential solution to this is metabolic funneling, producing a limited number and type of platform chemicals that can be catalytically upgraded to a diverse array of products. Current limitations of metabolic funneling in plant cells include: i) poorly characterized metabolic pathways; ii) relatively slow phenotypic and metabolic analyses; and iii) the need for large multigene constructs. In addressing both challenges, we seek to accelerate the design-build-test-learn (DBLT) cycle in plant lignin engineering with ready applications to other lignin monomer-derived metabolites. We are currently interested in a renewable, sustainable source of aromatic chemicals.
Experimental Collaborator: Laura Bartley
In silico engineering of heterocyst-forming cyanobacteria for inducible carbon dioxide and light to bioproduct platforms
Human-caused climate change is threatening the global ecosystem with myriad political, human, economic, and ecological effects stemming from these changes. A popular idea to mitigate further climate change is the development of a circular carbon economy, for which carbon capture and upcycle technology will play a key role. The development of carbon-negative platforms to produce biofuels or chemical feedstocks which replace petrochemicals will be one key carbon capture technology. Cyanobacteria are emerging chassis for carbon-neutral bioproduction due to key advantages such as higher photosynthetic efficiency, ease of genetic manipulation, and being prokaryotic. Diazotrophic (nitrogen-fixing) cyanobacteria can be a CO2 to biochemical chassis which requires few inputs. As nitrogen fixation is strongly inhibited by oxygen, in some species specialized, terminally differentiated, heterocyst cells to create anoxic environments. While often slow-growing, it offers the opportunity of an inducible system, as nitrogen starvation induces cell differentiation, and large changes in transcriptional regulation which could be harnessed to bioproduction without forsaking photosynthesis. Our research aim is to incorporate diffusion limitations into metabolic modeling to develop cyanobacteria as a self-catalyzing system for carbon and nitrogen compound production.